This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
WHAT IS A LIQUID STICK-PACK?
The stick-pack is a unit dosage form that can be used to contain and deliver liquid health products for oral and topical use.
The packaging protects the contents from moisture, oxygen and light. Thanks to its small size and compactness, the unit-dose is user-friendly and very practical for everyday life.
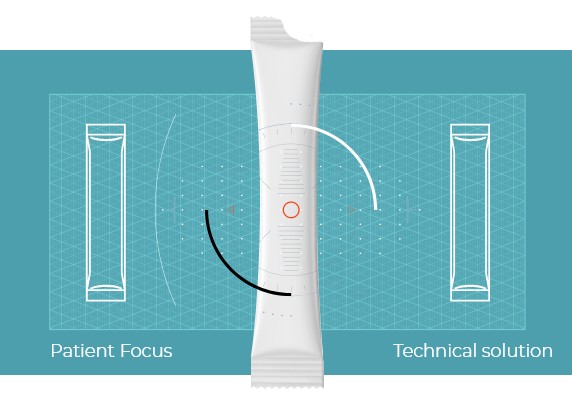

PATIENT BENEFITS
- “I have been waiting for this type of product on the market for a very long time. When you travel, when you have to go out, it is absolutely ideal. It allows you not to interrupt a treatment, simply because the usual product cannot be carried.”
- “These ready-to-use formulas are suitable for everyone: children, teenagers, pregnant women and older people.”
READY TO
USE
68%
68% appreciate the convenience
of the form: ready-to-use,
which can be taken
anywhere and
at any time.
THE RIGHT DOSE
FOR EVERYONE
75% – three quarters (¾)
of the population would be willing to purchase ready-to-use forms for their loved ones (including children and seniors).
Source : National representative study in 5 EU countries (1000 persons by country), GFK and Unither, 2018
BENEFITS OF THE LIQUID STICK-PACK:
- Safety of use
- Delivery of the right dose
- Ready-to-use, robust and easy to carry
- Good product conservation
- Improved formulation (taste masking, and less or no preservatives)
- Possibility for the patient to purchase a stick-pack by unit
UNISTICK®: STICK-PACK BY UNITHER
The stick-pack is suitable for packaging a wide range of health products in drinkable or topical liquid form.
1 ml to 15 ml
Solutions, suspensions, emulsions and gels
Assembly of several components to guarantee and ensure the stability of the product over time
Drugs, Medical devices, Food supplements, Cosmetics
1 to 1500 cP
Suitable for all therapeutic areas (e.g. analgesics, gastroenterology, cardiovascular diseases, central nervous system, orphan diseases, cough and cold, etc.)
Individual stick-pack packaging helps to preserve products that are sensitive to oxygen (preparation and filling under nitrogen) or to light thanks to its barrier effect. Certain formulation options exist such as taste masking or preservative-free formulation.
OUR SITES
Unither Pharmaceuticals is a CDMO specializing in liquid stick-packs, offering support from formulation to industrial scale-up. The unique expertise of our multidisciplinary and international teams will meet all regulatory, development and manufacturing needs in France, the United States and Brazil.
COLOMIERS (France)
- Capacity: 420 million sticks / year
- Filling: 1 ml to 15 ml
- Lines: 4 (1 pilot line and 3 industrial lines)
- Authorizations: ANSM, EMA, Anvisa, KFDA, Health Canada, MHRA, South Korea, etc.


ROCHESTER (United States)
- Capacity: 250 million sticks / year
- Filling: 2.5 ml to 10 ml
- Lines: 2 (1 pilot line and 1 industrial line)
- Authorizations: MHRA, FDA, ANVISA, Health Canada, Turkey, Mexico, etc.


BARRETOS (Brazil)
- Capacity: 70 million sticks / year
- Filling: 5 ml to 15 ml
- Lines: 3 (1 industrial line and 2 pilot lines)
- Authorizations: ANVISA


COLLABORATIONS ALL OVER THE WORLD
100
SKUs
+30
Customers
50
Countries where
our products
are sold
Two types of collaboration:
We offer our customers two types of collaboration: the
development and manufacturing of new products based
on their specifications, and/or a turnkey product from our product list.
Contract development
and manufacturing
for strategic subcontracting
Turnkey products and dossiers
for licensing-in
